REGULATORY ASPECTS, QUALITY AND SAFETY ISSUES OF NANOPHYTOMEDICINES: THE STATE OF ART
GIULIA VANTI, LUCIA GRIFONI, ANNA RITA BILIA | Nanomedicine is associated with nanopharmaceuticals having nanoscale structures, developed with the aim to improve the physicochemical, biopharmaceutical, and pharmacokinetic properties of drugs. The past decades have been successful in placing on the market several nanopharmaceutical formulations for the treatment of diverse pathologies, principally cancer and infectious diseases.
In recent times, nanotechnologies have been revealed also crucial in developing appropriate dosage forms of emerging natural constituents and extracts with pleiotropic functions for future therapeutic treatments.
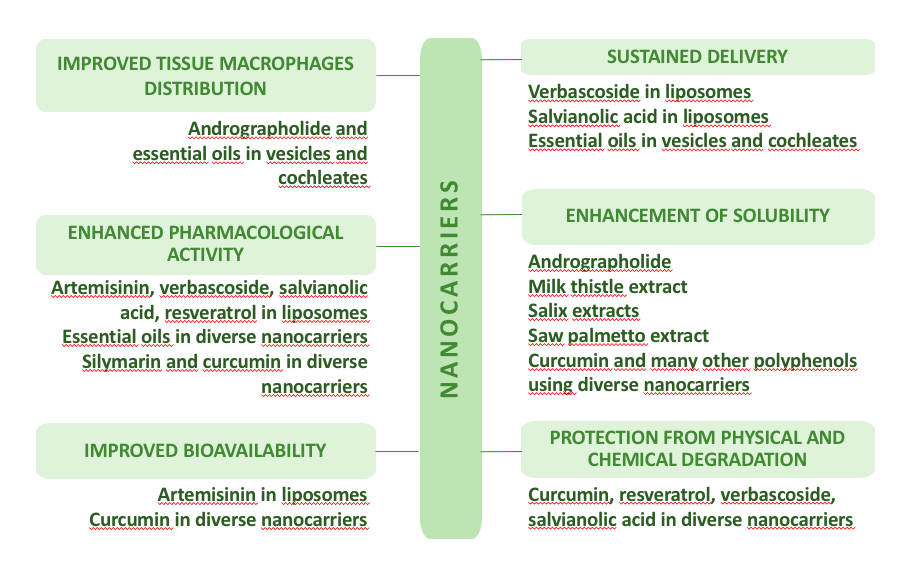
Nanosized phytomedicines can successfully increase selectivity and activity, protect against thermal- or photodegradation, reduce side effects, promote sustained release of active constituents, or reduce the required dose, generally resulting in improved activity, grabbing future prospects of nanoformulations of phytoconstituents [1,2], see figure 1.
Scientific literature reports a huge number of different nanoformulations based on extracts or pure natural products, under pharmacological or clinical studies with potential translation into commercial products. Their potential use focuses on the areas of herbal medicinal products, functional foods and food supplements, but in Europe nanophytomedicine have been marketed within the last two categories.
Regulations
Nanophytomedicines and nanoformulations in general have exceptional, unique, distinctive and singular characteristics associated with their size, matrix composition, and surface properties, with respect to the traditional herbal medicinal products (HMP). In Europe, the current legislation cannot attend the needs of these new types of pharmaceuticals, and due to the lack of specific regulations some “Recommendations” and “Reflection papers” have been developed in the last two decades [3].
In 2011 the European Commission has defined the term “nanomaterial” as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1 and 50%” [4]. However, in pharmacy, particles of 10–500 nm have been used, rarely up to 700 nm. From the aspect of passage through vessels, the inside diameter of which is in the range from 25 mm (aorta) to 5 μm (capillaries), the ideal size of nanoparticles should be <300 nm to ensure efficient transport for targeted distribution of drugs [1,2].
To assist pharmaceutical industry to submit the required documentation for the market authorization for nanomedicines, and to clarify certain scientific doubts, the European Medicines Agency (EMA) published in 2006 the first regulatory reflection paper on nanotechnology–based medicinal products for human use [5].
In 2009 EMA established the European Nanomedicines Expert Group composed of high profile academics and regulatory science specialists [5]. The expert groups has interacted with regulatory scientists from the US Food and Drug Administration, the Japan PMDA and other regulatory agencies to discuss the expectations on the quality, non-clinical and clinical data to be submitted in support of a marketing authorisation application of specific classes of nanomedicines.
Up to now, EMA has released four reflection papers related to block copolymer micelle products [6] and to the coating of nanomedicinal products [7], as well as to intravenous liposomal products [8], and iron‐based products [9] developed with reference to innovator products.
Pharmaceutical companies have the support to market nanoformulations by the EMA Scientific Advice, Innovation Task Force and the newly established EU Innovation Offices Network (European Union Medicines Agencies Network Strategy to 2020 – Working together to improve health).
The increasing marketing of nanocarriers and nanostructures in food has required a revision of the regulation also in food and feed product areas, in particular for those products containing structures at the nanoscale in conventional materials that do not meet the definition of engineered nanomaterial as set out in the Novel Food Regulation (EU) 2015/2283 [10]. In this Novel Food Regulation ‘engineered nanomaterial’ is defined as any intentionally produced material that has one or more dimensions of the order of 100 nm or less or that is composed of discrete functional parts, either internally or at the surface, many of which have one or more dimensions of the order of 100 nm or less, including structures, agglomerates or aggregates, which may have a size above the order of 100 nm but retain properties that are characteristic of the nanoscale.
The Guidance on Particle-technical requirements complements the Guidance on Nanoscience and Nanotechnology [11] adopted by the EFSA Scientific Committee in 2018. This guidance sets out information requirements for applications in the regulated food and feed product areas, and establishes criteria for assessing the presence of a fraction of small particles [12]. This document represents a revision of the previous Guidance on risk assessment of nanoscience and nanotechnology applications in the food and feed chain published in 2011 [13]. The Guidance has taken account of relevant new scientific studies that provide more insights to physicochemical properties, exposure assessment and hazard characterisation of nanomaterials. It specifically elaborates on physicochemical characterisation of nanomaterials in terms of how to establish whether a material is a nanomaterial, the key parameters that should be measured, the methods and techniques that can be used for characterisation of nanomaterials and their determination in complex matrices. It also details the aspects relating to exposure assessment and hazard identification and characterisation. In particular, nanospecific considerations relating to in vivo/in vitro toxicological studies are discussed and a tiered framework for toxicological testing is outlined. The Guidance proposes approaches to risk characterisation and uncertainty analysis, and provides recommendations for further research in this area.
Quality
The assessment of the quality of the pharmaceutical product including physicochemical characterization, stability, purity, and sterility of the formulation is an indispensable part of the preclinical evaluation. The relevant guidelines on quality are provided by the International conference on harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use, however, there are no specific ICH guidelines for the nanosized formulations. By contrast, some aspects can be crucial for the quality and reproducibility, mainly represented by their physical and chemical characterization covering a series of properties such as size distribution, shape, surface coating and charge, hydrophobicity, chemical composition, dispersion, stability, and others. Their evaluation can be particularly complex and additional tests should be carried out in the relevant biological medium, i.e. blood, gastric or intestinal environments etc., because the presence of solutes or macromolecules (principally proteins) in the physiological media can modify characteristics of nanosized formulations. As a consequence, also processes such as release, stability, dissolution, formation of a protein corona can be strongly affected and their properties changed profoundly [14].
Up to date, also the European Pharmacopoeia do not contain the methods specifically addressing nanotechnology‐based products. However, several techniques for particle size measurement such as dynamic light scattering, laser light diffraction or image analysis are relevant and currently used for the characterization of nanomedicines. Consequently, the producers of nanosized medicines can use the standardized ISO methods [15]. However, their suitability for testing nanomedicinal products should be proven.
Finally, a crucial property of nanomedicines is the efficiency of drug loading and the ratio of carrier‐bound or encapsulated drug to free drug after purification, affecting dramatically the quality, the efficacy and safety profile of the product. Additionally, the possibility that (in vivo) the free drug is in equilibrium with the protein‐bound drug (i.e. albumin) and as well with the drug in its nanoformulation adds a further challenge to this task [16]. For those products having active targeting to particular diseased tissues (i.e. cancer, inflammation), the carrier should ideally deliver the drug directly to the diseased cells leaved unharmed the others. Thus, free drug in blood is a sign of imperfect targeting [16,17].
Once more, internationally accepted consensus guidelines on how to determine encapsulation efficiency and drug release from the nanoscale delivery systems do not exist, but due to the peculiarities of systemic release and targeting, new guidelines should be properly developed on the basis of existing ones dealing with modified‐release of pharmaceutical products [18,19].
So far, most nanomedicines on the market are based on liposomal carriers. Methods to determine liposomal encapsulation in scientific studies generally efficiently start with the separation of the encapsulated drug from the free drug by chromatographic techniques, centrifugation, (ultra)filtration or dialysis, followed by analysing both fractions separately, and quantifying the drug after destruction of the liposomes [20].
Safety
Actually, there is an increasing number of studies on nanophytomedicines, performed at basic and clinical trial levels. Generally, the nanotechnologies generate new insights and developments, which are accompanied by new challenges. The use of nanoparticles has raised safety concerns and the term “nanotoxicology” has emerged in recent years because the transport, metabolism, excretion, and immune response mechanisms of nanoparticles may differ significantly from those of traditional forms. Both engineered nanoparticles or the products and materials that contain them need to receive special regulation regarding production, handling or labelling, integrated more than before.
The guidelines for the preclinical safety assessment, released by the International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use (ICH 2009), are now applied also to candidate nanomedicines. However, since developed for small molecule drugs, they may not be suitable to address nano‐specific safety issues [21]. In addition, nanomedicines might trigger particular effects in biological systems, which have to be evaluated in addition to standard toxicity studies.
Adverse immunological effects
Effects on hematotoxicity, antigenicity, and immunotoxicity including complement activation, should be considered in the preclinical development for liposomes and polymeric micelles according to EMA reflection papers [6,8]. Many researchers have pointed out the complement activation, cytokine release and other immunological and hematological reactions, by the nanoparticles, leading to a compromised safety and efficacy. In a recent review, the activation of the immune system, including the activation of macrophages and the release of cytokines, is reported to be the most frequent effect of preclinical studies with nanomaterials observed in vivo. The majority of the investigated studies (about 60%) demonstrated an interaction with the immune system either in terms of stimulation (47%), suppression (10%) or dysregulation (2.5%), covering the ambiguous effects on the immune system. In 40% of the studies, no significant effect on the immune system was found. Inorganic nanoparticles (NPs) were responsible for 68% of all described adverse effects on the immune system.
However, activation of immune responses gives inflammatory reactions, which were almost exclusively induced by inorganic nanoparticles, mainly carbon-based or metal-based nanoparticles, in particular, containing titanium dioxide, gold, silica and graphene oxide–based nanomaterials [22].
The reflection papers issued by the European Medicines Agency in 2013 related to information needs for intravenous liposomal products, iron-based products and block copolymer micelle products recommend additional immunotoxicity studies including complement activation and macrophage activation, antigenicity and hypersensitivity reactions using validated in vitro and in vivo methods in sensitive models.
Up to now, there is only one standardized method for the evaluation of immunological response to nanoparticulate materials, developed by the American Society for Testing and Materials [23]. Another ASTM method refers to in vitro assessment of the whole complement activation by solid materials used in medical devices [24]. However, it does not address medicinal products or products based on nanotechnology. Complement activation, secretion of proinflammatory cytokines and leukocyte proliferation are part of the assay cascade provided by the European Nano-medicine Characterization Laboratory (EUNCL) for the preclinical characterization of nanomaterials intended for medical use [25]. More efforts are needed to develop and standardize test methods for assessment of the immunological response to nanotechnology-based pharmaceuticals.
Similarly, the formation of protein corona as the effect of the interaction of nanoparticles with the blood proteins was shown to highly influence the effect on the immune system [26,27], even if the exact molecular mechanism is not known up to now. In the recent guidance released by the Food and Drug Administration (FDA) the analysis of the protein corona is recommended for the drug products that contains nanomaterials [28].
Although toxicity is mainly related to the inorganic nanoparticles, it is well known that the nature of the lipids or polymers can strongly affect the safety profiles. Another important mechanism of immune activation is the anti-PEG response, which occurs when pegylated nanoparticle formulations activate the production of PEG antibodies by B lymphocytes. This leads to recognition and clearance by the immune system.
Liposomes and micelles represent the majority of the nanomedicines available on the market. They are the most investigated safe nanocarriers, because they reduce systemic toxicity from the encapsulated agents. However, they have a certain in vivo toxicity when formulated as cationic micelles or liposomes which are largely studied for gene delivery. Cationic liposomes and micelles are known to elicit toxicity in macrophages, macrophage-like cells, and monocyte-like cells, as well as alter the secretion of important immuno-modulators [29,30].
Improving bioavailability
One of the most interesting characteristic of nanoparticles is their ability to overcome physiological barriers, which hinder the absorption and permeation of drugs into the body, thus influencing the bioavailability of therapeutics, see figure 2. P-glycoprotein (P-gp) is one of such barrier present on the apical membranes of various organs such as small intestine, brain, kidney and liver. This protein interacts with a vast variety of therapeutics and efflux them out, preventing their entrance to the desired site, thus modulating their pharmacokinetic properties. To address this, a large number of approaches have been used for nanomedicines. The substances able to prevent drug efflux are represented by a large plethora and include surfactants (non-ionic surfactants including Tween 20, Tween 80, Myrj 52 and Brij 30), natural polymers (xanthan gum, sodium alginate, gellan gum) and synthetic PEG 400, 2000 and 20000 which can form micelles as in the case of poly(ethylene glycol) 2000–phosphatidyl ethanolamine conjugate (PEG2000–PE) and D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) [31].
It is well known that nanoparticles, in particular those made of chitosan can manipulate tight junctions in the paracellular pathway enhancing the passage of the drug. Tight junctions are seals between adjacent epithelial cells restricting paracellular flux of water, ions and solutes and the majority of drugs. They also form a barrier that prevent the passage of inflammatory and infectious agents into the systemic circulation. According to some authors these nanoparticles may constitute a safety problem concerning integrity of tight junctions, but there is no evidence up to now [32]. Some studies on chitosan nanoparticles are encouraging to believe they are safe. Indeed, chitosan nanoparticles increased the permeability of model molecules by producing a reversible tight junction effect [33].
Finally, transcellular uptake of nanoparticles can also occur. It is well known that after oral administration, nanoparticles can penetrate the gut mucosa by transcytosis, a process by which carriers are taken up by enterocytes or M cells, mimicking the entry of pathogens, but also in this case no data concerning their possible toxicity have been reported [33].
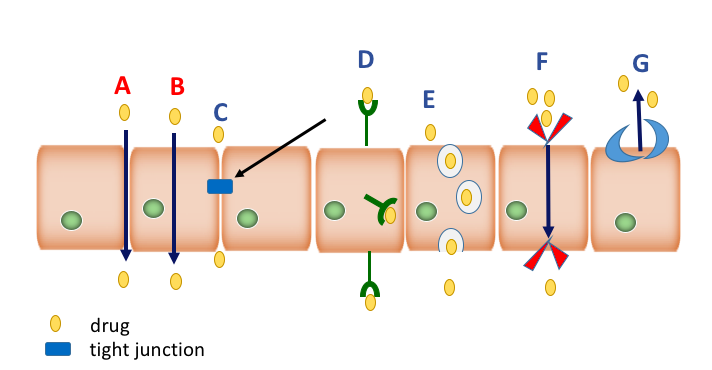
There are six main specific and non-specific mechanisms of penetration, consisting of passive and active pathways. Passive pathways consists of paracellular aqueous route (A) and the transcellular lipophilic route (B). Active pathways consist of tight junction modulation (C), transcytosis, the leading approach which consists of receptor-mediated transcytosis (D) and adsorptive mediated transcytosis (E), and carrier-mediated transport (F). Finally, efflux transporter proteins (G) provide important protection in the blood–brain barrier, intestines, liver, kidney, and placenta.
Very interesting, nanoparticles can also overcome the blood brain barrier (BBB), either through paracellular or transcellular pathways. A paper has investigated human serum albumin based nanoparticles (HSA NPs) and poly ethylcyanoacrylate nanoparticles (PECA NPs) which were loaded with the natural bioactive andrographolide, a major diterpenoid from Andrographis paniculata. Both types of nanoparticles were able to cross the blood-brain barrier (BBB) in an in vitro model, although the PECA NPs temporarily disrupted BBB integrity [34].
Furthermore, nanoparticles were able to pass the blood-brain barrier after systemic administration in rats. Lack of toxicity in C57/B6 mice was shown when these nanoparticles were chronically administered [35,36].
Also, in an Alzheimer’s disease mouse model it was shown that these andrographolide nanoparticles were able to cross the BBB and to penetrate in both undamaged and damaged brain tissues. Furthermore, evidence was obtained of anti-inflammatory activity in the hippocampus when andrographolide nanoparticles were intraperitoneally administered. These nanoparticles were non-toxic, non-immunogen, with a strong biocompatibility, and high biodegradability [37].
Conclusions
Nanomedicine is an emerging sector with great potential, progressing fast and using a variety of materials, coatings, and functionalization. The availability of robust and accepted testing methods is urgently needed for nanomedicines, from the laboratory environment to clinical applications, considering the multidisciplinary characteristics during early development of these systems.
Authors
Dr. Giulia Vanti (PhD) is experienced in development and characterization of nanosized drug delivery systems based on natural products.
Lucia Grifoni (MSc Pharmacy) has expertise in the development and evaluation of the biopharmaceutical characteristics of natural products loaded in microemulsions.
Prof. Dr. Anna Rita Bilia is full professor and Director of the Post-graduate School of Hospital Pharmacy. She is leading the group of natural products analysis and pharmaceutical technology at the Department of Chemistry “Ugo Schiff”, University of Florence. She is – amongst others – a member of the scientific committee of the ESCOP and of the TCM group of the European Pharmacopoeia and former president of the International Society for Medicinal Plant and Natural Product Research.
Correspondence
Prof. Dr. A.R. Bilia. Department of Chemistry “Ugo Schiff”, University of Florence, via Ugo Schiff 6, 50019 Sesto Fiorentino (FI).
ar.bilia@unifi.it







